In situ decellularization of tissues to resolve the tumour-associated matrix
Go behind the scenes with Alejandro Mayorca-Guiliani and discover a valuable tool for future extracellular matrix research in the nature reviews cancer “Tools of the Trade” article.
Filopodia rotate and coil by actively generating twist in their actin shaft
A recent paper on the fundamental functions of filopodia published in Nature Communications, with Lena Wullkopf and Janine Terra Erler as co-authors, is being featured in the Editors Highlights!

New disease model puts metastasis in a box
Cancer cells can travel away from the tumour where they originate and establish distant colonies (a process known as metastasis). Metastatic tumours are extremely difficult to treat and remain responsible for most cancer patient deaths. Researchers at the Biotech Research and Innovation Centre (BRIC) at the University of Copenhagen (UCPH) have succeeded in modelling the critical steps of metastatic tumour formation by designing a novel model system using native organ structural scaffolds, comprised of the extracellular matrix (ECM), published in Advanced Healthcare Materials (“Modeling Metastatic Colonization in a Decellularized Organ Scaffold-Based Perfusion Bioreactor“).
The model, developed in collaboration with clinical scientists from Copenhagen Hospital Region, uses an ECM scaffold from murine lungs, or livers (two frequent sites of metastasis), and challenges cancer cells to colonise and modify the ECM, mimicking the formation of metastatic tumours. The scaffold is housed in a box that provides circulation, nutrition and the possibility to monitor cells as they advance and conquer their new environment. Remarkably, the ECM scaffold induces cells to communicate and behave in ways that imitate tumours in an organism. This opens the possibility to study metastatic disease in a controlled system, performing experiments that cannot be carried out using experimental animals or traditional cell culture systems.
Learn more about the research by watching produced by University of Copenhagen:
“The key advantage of our model is flexibility of its applications”, said Dr Alejandro Mayorca Guiliani, the designer of the model system, “Metastasis is a threat to cancer patients and a very difficult phenomenon to study. Our model can use scaffolds from different organs and cells carrying different origin and mutations which opens new experimental possibilities to explore the mechanisms of metastasis and other diseases”.
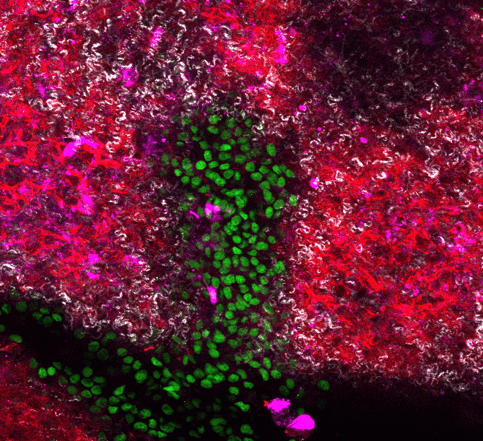
“Using this approach can help to identify and target key factors needed for metastatic tumour formation. It allows us to look at both sides of the process: how cancer cells respond to the ECM and how the ECM is transformed in cancer cells’ favour”, added first author PhD student Maria Rafaeva. One key finding of this study suggests that ECM scaffold can coerce cells to activate an important family of “druggable” proteins, tyrosine kinases, similarly to real tumours.
“Designing effective treatments against metastasis is an urgent task for cancer researchers” said Prof. Janine Erler, senior author of this study, “We hope our model system can become a tool to identify and test new therapeutic strategies against this major clinical problem.”
This work was funded by the European Research Council, Innovation Fund Denmark, Danish Cancer Society, German Cancer Aid, Novo Nordisk Foundation, Lundbeck Foundation, Danish Research Council, among other international agencies.
Promising study on the immune system in pancreatic cancer
New study by Erler group explores the immune system’s function in pancreatic cancer and finds that an existing therapy can be used to suppress pancreatic tumor development in pre-clinical mouse models. The research was supported by the Danish Cancer Society.

From left: Professor Janine Erler (senior author) and joint first authors PhD student Jan Strøbech & postdoc Sebastian Rune Nielsen
Pancreatic cancer is a devastating disease that is often detected at an advanced stage, which makes it very difficult to treat, and new therapeutic options are needed. Researchers at Biotech Research and Innovation Centre (BRIC) at the University of Copenhagen have now discovered a compound that could offer a new therapeutic option for treatment of pancreatic cancer.
The researchers have found that lorlatinib, which is already approved for the treatment of specific types of lung cancer, can suppress the development of pancreatic cancer in pre-clinical mouse models. Even though there is a long way from pre-clinical mouse models to treatment of patients, the results are encouraging according to the first author of the study, postdoc Sebastian Rune Nielsen.
“It is difficult to achieve a good response to treatment in mouse models of pancreatic cancer, but when we treat with lorlatinib we are able to suppress tumor development, and when we combine the drug with anti-PD-1 immunotherapy, we achieved an even better response with the combination,” he explained.
He is complemented by senior author Janine Erler, professor at BRIC.
“It’s encouraging that the drug is already approved for treatment of lung cancer. That means it has passed initial phases of clinical trials and that it is well tolerated by patients“, says Janine Erler.
Immature immune cells
Sebastian Rune Nielsen focuses on the role of the immune system in pancreatic cancer. He explains that this cancer type is characterized by a high degree of immune cell infiltration by cells from the innate part of the immune system, among them neutrophil granulocytes. They are produced in the bone marrow and serve as the immune system’s frontline soldiers.
“Neutrophil granulocytes are supposed to arrive quickly to a site of infection and neutralize the threat to make way for other immune cells that participate in the rebuilding of the infected tissue. But their role seems to be inverted in pancreatic cancer, and they promote rather than fight tumor development“, he explains and continues:
“Our hypothesis is that the tumor cells apply pressure to the bone marrow to promote the production of neutrophil granulocytes, which leads to the release of immature cells that are not functioning as they are supposed to. One can say, that pancreatic cancer corrupts the immune system to promote tumor development“, explains Sebastian Rune Nielsen.
Treatment blocks cell signaling
The main goal of the research was to find a way to modify the neutrophil granulocytes to make them fight the cancer rather than support its growth, continues Sebastian Rune Nielsen.
By searching through the scientific literature, the researchers found the drug lorlatinib. It is a tyrosine kinase inhibitor that can block a specific signaling pathway in the cells, FES, which the researchers found was activated in the neutrophil granulocytes by the cancer cells.
“We demonstrate in our experiments that lorlatinib blocks the signal that FES was supposed to send resulting in suppression of the neutrophil granulocytes“, explains Sebastian Rune Nielsen and continues:
“We demonstrate that treatment with lorlatinib reduces the production and release of neutrophil granulocytes from the bone marrow, which leads to reduced infiltration in pancreatic tumors. Our results also demonstrate that we can achieve better response to immunotherapy, when we combine lorlatinib with immunotherapy, so called anti-PD-1 blockade. This combination leads to less neutrophil granulocytes, but more active T cells, another type of immune cell, and further reduction in tumor sizes“, he explains.
The right combinations
Sebastian Rune Nielsen hopes that this study can improve the therapeutic options for treatment of pancreatic cancer.
“I believe that research in the future will be aimed at finding the right combinations of drugs. We have not seen good clinical responses to treatment with immunotherapy in pancreatic cancer, but we do get a good response in our experiments when we combine it with lorlatinib. But it is going to take a lot of work and research to find the best combinations“, says Sebastian Rune Nielsen.
This new study was published in the recognized scientific journal Nature Communications.
Contact:
Sebastian Rune Nielsen: Sebastian.Nielsen@bric.ku.dk
Researchers at BRIC, University of Copenhagen, uncover a novel way to improve pancreatic cancer treatment
Nielsen et al, Nature Communications 2021
Pancreatic ductal adenocarcinoma (PDAC) patients have a 5-year survival rate of only 8% largely due to late diagnosis and insufficient therapeutic options. In their study, the researchers from the Erler Group at the Biotech Research and Innovation Centre (BRIC) at the University of Copenhagen, uncover a novel therapeutic approach to slow down PDAC cancer progression.You can find the published article here.
The researchers demonstrate, using pre-clinical mouse models of PDAC, that lorlatinib, an FDA-approved drug from Pfizer for treatment of certain types of lung cancer, suppress PDAC cancer progression. They further show that combining lorlatinib treatment with immunotherapy improves the anti-cancer effects of anti-PD-1 blockade.
“Our results strongly suggest lorlatinib treatment could extend pancreatic cancer patient lives. Excitingly, lorlatinib is already approved for clinical use so we hope our studies can be quickly applied to human patients.” explained Professor Janine Erler.
The study reveals that lorlatinib targets neutrophils, which are immune cells involved in promoting cancer progression.
“Our study has uncovered a way to specifically target tumor-associated neutrophils, which has great therapeutic potential as neutrophils are known to promote the progression of many cancer types.” added first author Dr Sebastian Rune Nielsen.
The authors further confirmed their findings in a pre-clinical model of colon cancer and expect the findings to be relevant to other solid types of cancers.
The study was predominantly funded by the Danish Cancer Society and the European Research Council, and involved international collaborators from Scotland and the United States of America.
New scientific discovery may enable accurate prediction of cancer spread even before cancer develops
Researchers from Erler Group at the Biotech Research & Innovation Centre (BRIC) in Copenhagen have discovered that the rigidity of a thin membrane structure encompassing cells and lining all vessels regulates how easily cancer cells can breach tissues to spread through the body, and is thus a key determinant of cancer patient survival. The results are published in Nature Materials today. You can find the published article here.
The researchers analysed cells, mouse models, and human patient samples using biochemical, mathematical, and biophysical methods. They identified a protein present in the mesh-like membrane structure (the basement membrane) associated with tumour and vessel softness, and good survival of cancer patients. The researchers tested if removing this protein from the basement membrane would enhance the spread of cancer, which it did, and if supply of this protein would reduce cancer spread, which it did. They proceeded to show that the levels of this protein (netrin-4) already present in basement membrane of organs may determine cancer spread even before cancer develops, in several cancer types.
“These are extremely exciting findings that open up the possibility to predict which organs a person’s cancer most probably spread to before they even have cancer. This information therefore has the potential to guide and improve cancer patient treatment and care.” said Professor Janine Erler, senior researcher on the paper.

LEFT: Spheroid of breast cancer cells breaking through basement membrane to invade into the surrounding area. This ability is determined by the ratio of netrin-molecules present in the basement membrane composition (image from Erler lab). RIGHT: Schematic to explain the impact of basement membrane softness on cancer cell’s ability to cross vessels.
In their study, the researchers could show for the first time the impact of basement membrane composition on its mechanical properties thereby affecting cancer cell transmigration over this protein border within the inter-cellular space. They identified the secreted protein netrin-4 to open nodes inside the basement membrane network, which simultaneously softens the basement membrane and increases its mesh (pore) size. This unexpected finding highlights that cancer cell movement is dominantly controlled by the basement membrane stiffness and pore size plays an underpart. Thus, the more netrin-4 inside the basement membrane of the primary tumour or inside blood vessels within organs prone to metastasis, the less metastasis, impacting on patient survival. Moreover, they demonstrate for the first time that the mechanical properties of the basement membrane independent of cancer-related modifications are a pivotal determinant of cancer patient survival.
“We were incredibly excited to find a mechanistic explanation for our observations where the theoretical modelling closely matched our experimental data. We could show that the more netrin-4 molecules present, the softer the basement membrane and the more difficult for a cell to traverse this membrane thereby keeping cells contained in one area. We are currently exploring the therapeutic and diagnostic potential of our findings. Our study is a result of a huge collaboration effort from researchers in Denmark, Sweden, Germany, the UK, and Belgium, spanning many disciplines, which has been key to obtaining the exciting results.” added Dr Raphael Reuten, lead researcher on the study.
Historically, there has been much focus on the stiffness of the extracellular matrix that lies outside of cells and the influence on cancer progression. However, there have not been studies investigating the influence of basement membrane stiffness. Moreover, studying the impact of mechanical properties of the basement membrane on cell invasion and cancer metastasis has not been possible so far. The mechanistic insights into netrin-4 activity inside basement membranes has enabled the researchers to bridge this gap of knowledge and present new opportunities to study basement membrane stiffness in a broad range of biological processes.
This work was funded by the European Research Council, Danish Cancer Society, German Cancer Aid, Novo Nordisk Foundation, Lundbeck Foundation, Danish Research Council, among other international agencies.
New technique to unveil matrix inside tissues and tumours
We have developed a groundbreaking method to reveal the structure of tissues and tumours with unprecedented detail, by completely dissolving away cells and leaving the delicate extracellular matrix intact.
The matrix surrounds the cells in every organ of our bodies, and provides shape and structure to the organ. The matrix has a profound impact on how cells behave, and so controls the progression of diseases such as cancer. Yet the matrix is extremely difficult to study in detail.
Now our team has developed a new technique published in Nature Medicine today that makes closer study of the matrix possible. The technique, which is known as ‘ISDot: in situ decellularization of tissues’ reveals the inner structure of organs and tumours by removing cells but leaving the matrix completely unaltered. The three-dimensional structure of this matrix has never been seen in such detail before. You can find the published article here.
“We have developed a technique to obtain intact organ scaffolds and to image them in incredibly high detail using microscopes. We are the first to image the 3D structures of primary and metastatic tumours as well as healthy organs in this way”, says Professor Erler.

A world of details revealed
Cells which are organised together to form tissues rely on the extracellular matrix as a foundation for attachment, to arrange themselves properly, and to sense how to behave when their environment changes. Sometimes this organisation goes wrong and cells grow into tumours. To destroy a tumour, it is essential to know both its structure and the foundation upon which it is built.
The new method was pioneered by postdoctoral fellow Dr Alejandro Mayorca-Guiliani, who says, “We have isolated the structure that keeps tissues in place and organises the cells inside them. We did this by using existing blood vessels to deliver cell-removing compounds directly to a specific tissue to remove all cells within an organ. Doing this leaves behind an intact scaffold that could be analysed biochemically and microscopically, providing us with the first view of the structure of tumours.”
Imaging expert and co-first author Chris Madsen (now at Lund University, Sweden) says “When you remove the cells, the clarity of what you can see through the microscope is much improved – you can see the fibres of the matrix more clearly and you can look much deeper into the tissue. Using this approach, we have been able to see important differences in matrix organisation when we looked at metastatic tumours in the lung and in the lymph node.”
Matrix Biology and mass spectrometry expert and also co-first author Thomas Cox (now based at the Garvan Institute of Medical Research, Sydney) says “Because we are removing the cells completely, we can use mass spectrometry to identify and catalogue the components of the matrix – in normal tissue and in tumours – in unprecedented detail. What is really exciting is we found that some of the components of the matrix in different secondary tumours metastases are unique to that tissue. That is telling us that remodelling of the matrix in cancer is organ-specific”.
Understanding cancer progression
This research is an advance in the fields of both cancer research and bioengineering: By using the decellularised organs we can learn much more about how tumours and normal organs are built, and what their differences are. This new technique might evenhave an impact on organ regeneration and tissue engineering in the future.
“We are now re-introducing cells into our extracellular matrix scaffolds, bringing them back to life, to study how tumours form and how cancer progresses. This is extremely exciting and offers a unique opportunity to study how cells behave in their native environment,” explains Professor Erler.
The research is supported by the Danish Cancer Society, an ERC Consolidator Award, the Novo Nordisk Foundation, a European Research Council Consolidator Award, the Ragnar Söderberg Foundation Sweden, Cancerfonden Sweden, the Innovation Foundation Denmark, the National Health and Medical Research Council (NHMRC) Australia and the Danish Council for Independent Research.